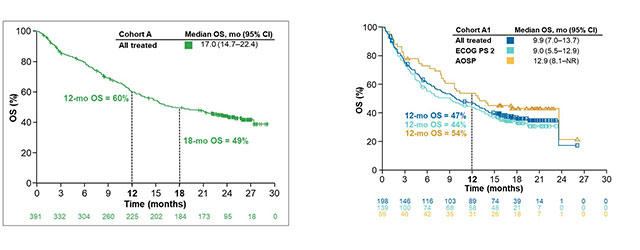
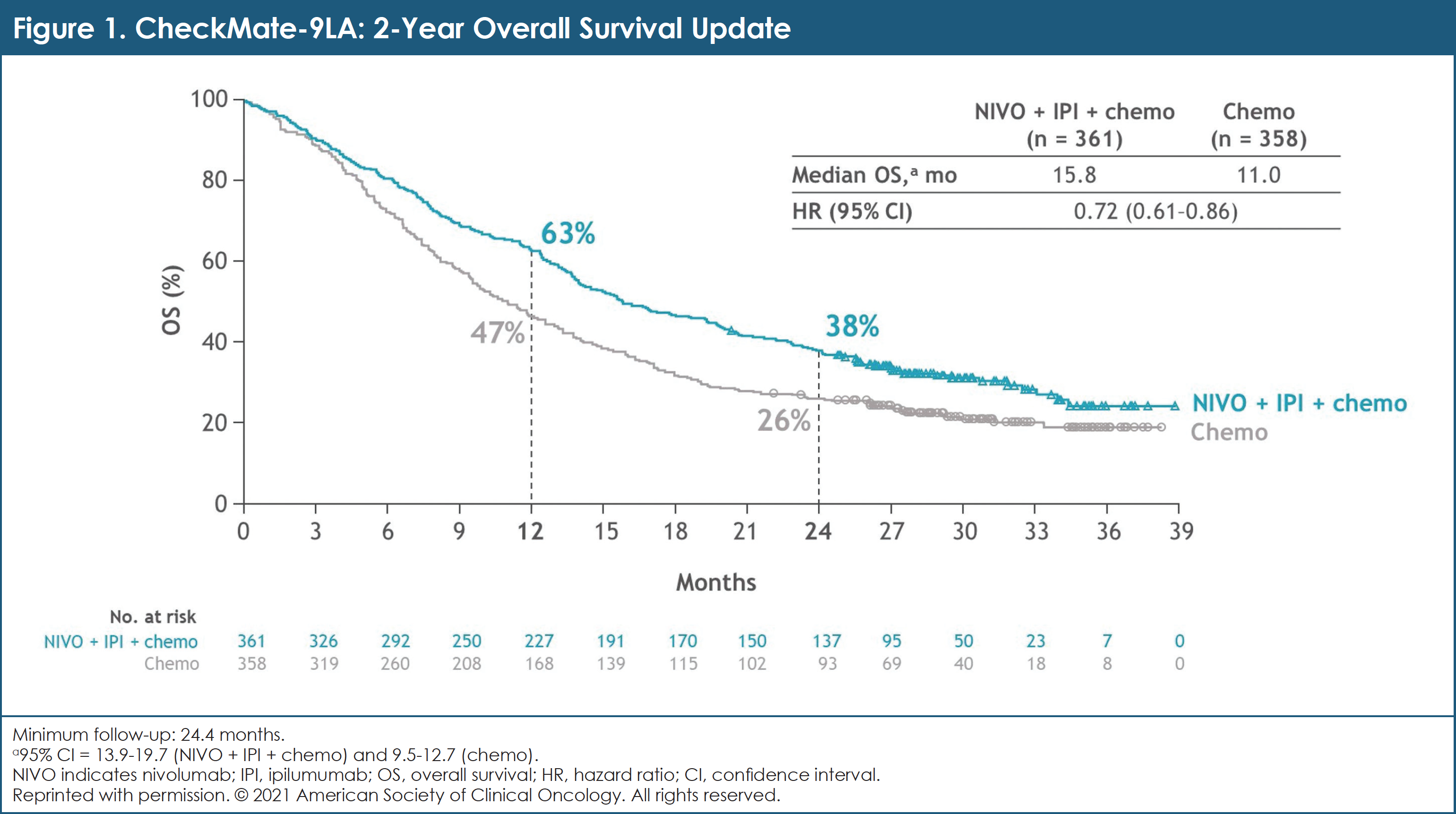
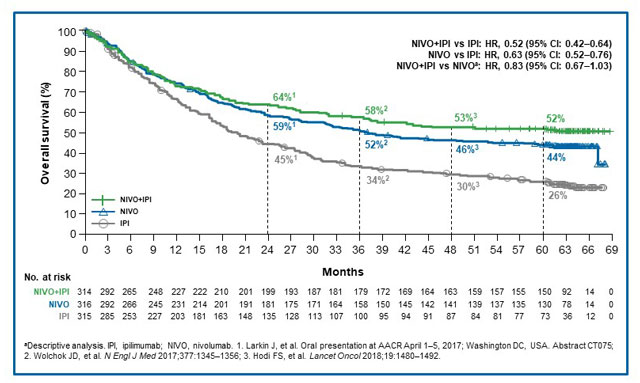
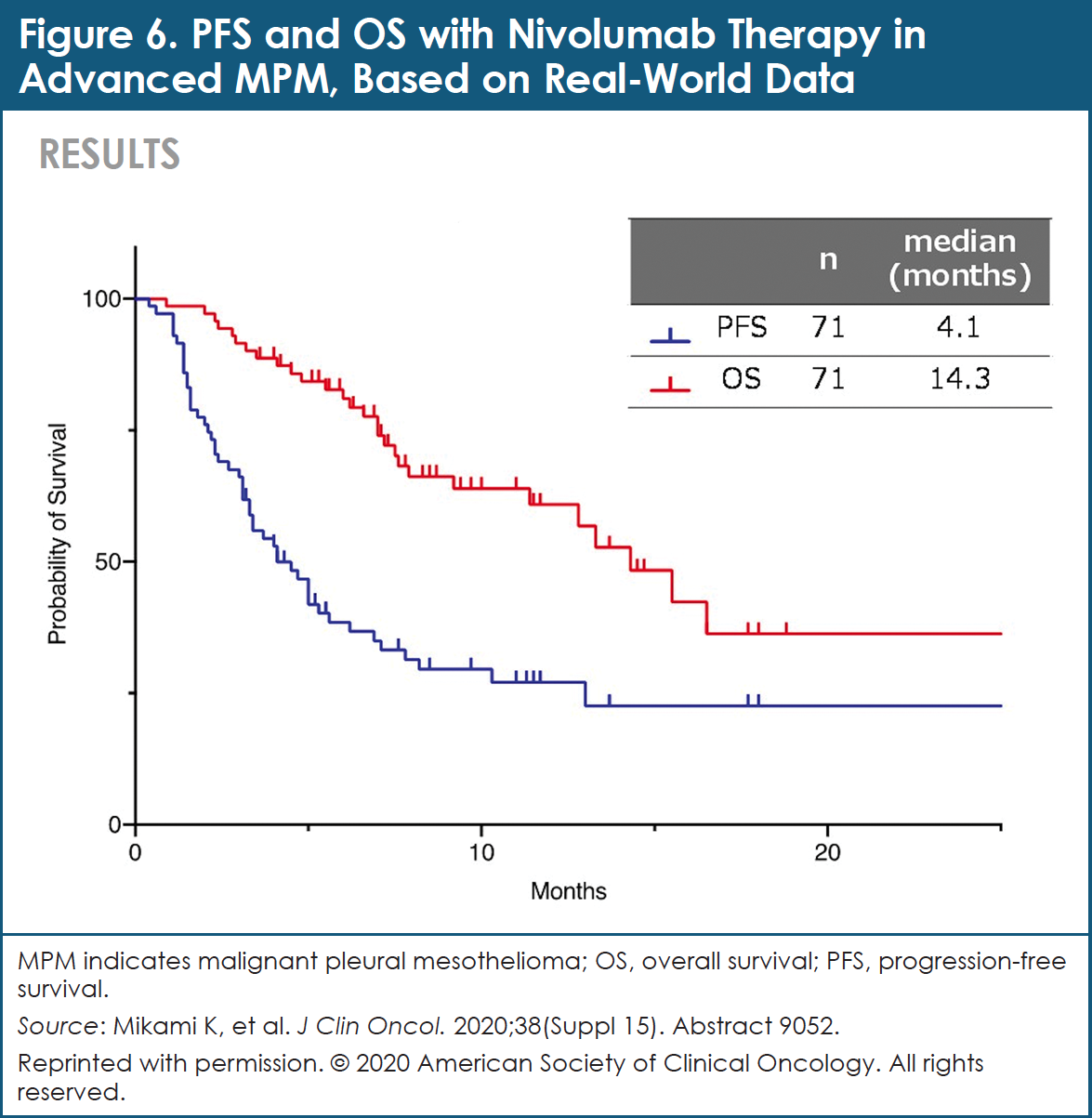
First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update - ESMO Open

First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial - The Lancet Oncology

Immunotherapy Advances - ASCO 2020: Key Lung Cancer Studies - Lung Cancer - 2020 ASCO Annual Meeting - Oncology - Clinical Care Options

First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial - ScienceDirect

FDA approves immuno-oncology combination as first-line treatment in patients with NSCLC who express positive PD-L1 greater than 1% - Onco Americas

Comparisons between the CheckMate 227 trial and simulation, overall... | Download Scientific Diagram

New CheckMate-227 5-Year Data Show Substantial Survival Benefit of Combination Immunotherapy for Metastatic Non–Small Cell Lung Cancer

ISPOR 2022: Mixture-Cure Modelling of the Overall Survival Outcomes of Patients with Advanced Non-Small Cell Lung Cancer (ANSCLC) Receiving Nivolumab + Ipilimumab in CheckMate-227

Frontiers | Cost-Effectiveness of Nivolumab Plus Ipilimumab With and Without Chemotherapy for Advanced Non-Small Cell Lung Cancer

Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis - The Lancet Oncology
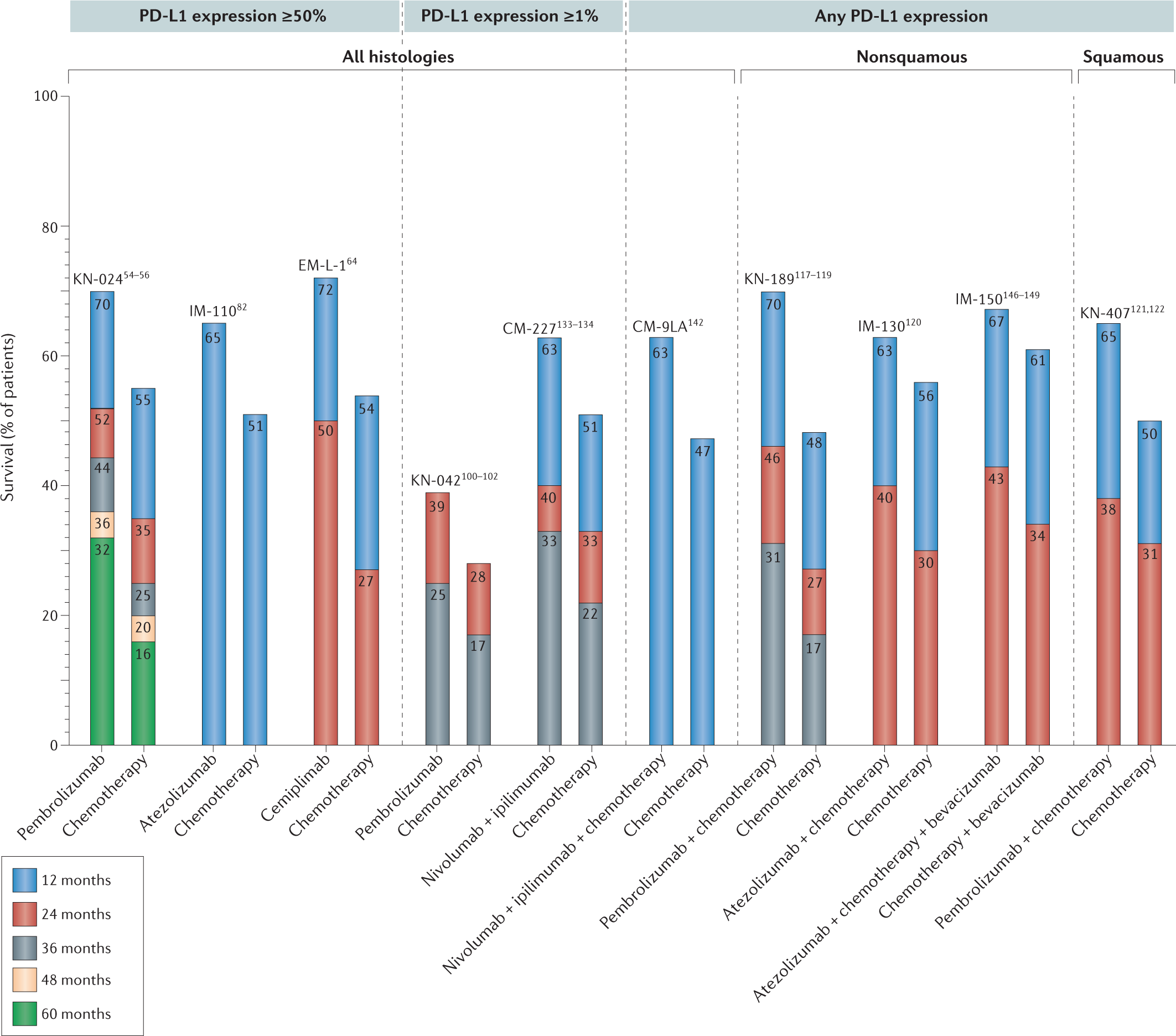
Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC | Nature Reviews Clinical Oncology
Kaplan-Meier curves after matching adjustment for (A) overall survival... | Download Scientific Diagram

Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non-small-cell lung cancer with high tumour mutational burden: patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. | Semantic

ASCO 2022: Five-year survival outcomes with nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first-line (1L) treatment for metastatic non–small cell lung cancer (NSCLC): Results from CheckMate 227.